Haemodynamics in human heart
- contact:
- funding:
Bundesministerium für Bildung und Forschung
BMBF-Projekt:
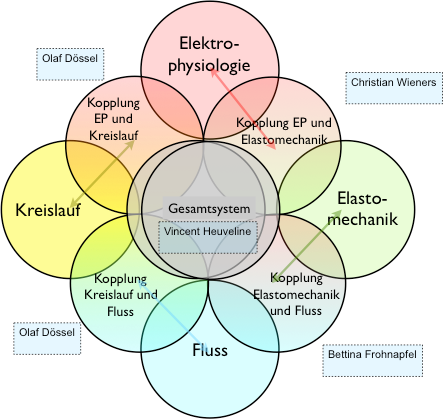
Ein integriertes Herzmodell - Kopplung von Elektrophysiologie, Elastomechanik, Fluss und KreislaufVerbundprojekt 05M2016
- Partner:
Prof. Dr. Olaf Dössel, Institutsleiter, Institut für Biomedizinische Technik (IBT), KIT
Prof. Dr. Christian Wieners, Institutsleiter, Institut für Angewandte und Numerische Mathematik (IANM), KIT
Prof. Dr. Vincent Heuveline, Institutsleiter, Engineering Math. and Computing Lab (EMCL)
Interdisciplinary Center for Scientific Comp. (IWR), Universität Heidelberg - startdate:
Januar 2017
The heart is the pump, that keeps our circulatory system moving and as cardiovascular deseases are nowadays one of the major causes of death, it is worth to gain a better understanding of each heart component, but especially of the interaction between the involved disciplines. Thus, the goal of the BMBF granted projects is to couple electrophysiology, mechanical engineering and fluid dynamics to an integrated full heart model. Therein, it is of major interest to determine the necessary coupling degree between the single disciplines facing the challenge of patient-specific accurate results at low computational costs.
Four institutes from two universities are involved, of which each brings expertise from earlier studies into the project. Obviously the ISTM focusses on the blood flow in the heart chambers. It interacts with the circulatory system through acting pressures and blood flow rates. The contraction of the heart muscle which drives the flow, is triggered by electromechanical activation. Thus, four components influence the pumping efficiency of the heart, from which two are directly linked with the fluid flow. We aim at developing efficient coupling procedures that enable a patient-specific modeling at low computational costs.
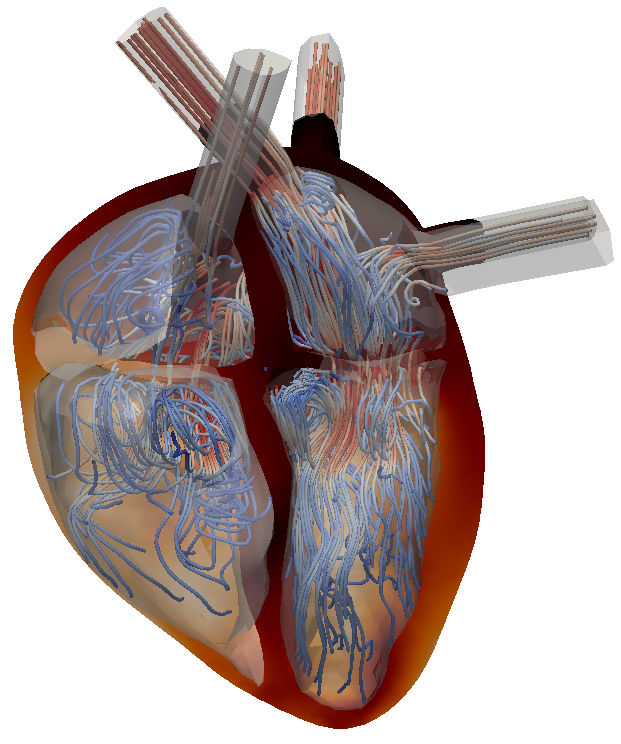
Approaches for modelling the heart valves are developed and tested with respect to their influences on the flow field structures. During the inflow phase of the blood from the atrium through the mitral valve into the left ventricle, the characteristics of the flow are primarily driven by the formation of a vortex ring.
If the heart is healthy, the vortex formation enhances a high level of mixing and a short particle residence time in the ventricle, while recirculation zones that remain in a diseased heart over a certain period of time may result in thrombus formation.
As such, it is beneficial to identify such recirculation regions and in turn predict flow modifications of upcoming surgical procedures. In order to evaluate the performance of an undertaken numerical surgery outcome, a full characterization of the flow field is essential. Primarily used methods are the evaluation of streamlines, the Q-criterion, particle residence times and the extraction of Lagrangian coherent structures.
Figure: Left and right ventricular flow during early diastole (inflow phase) visualized with 3D streamlines.
